
Gene drives are a class of biotechnologies with the potential to transform pest control in U.S. and global agriculture over the next decade. These technologies are being designed to permanently alter or eliminate sexually reproducing pests through the release of a relatively small number of genetically modified (GM) organisms. Prototype gene drives work by using a new, low-cost method for editing genes, called CRISPR/Cas9. With this method, scientists can insert or remove genes in a pest’s DNA in a way that ensures that, when the GM pest mates, all of its offspring will inherit the target gene. This feature means that a desired genetic trait can be driven through an entire pest population via an evolutionary “chain reaction” after releasing only a few of the GM individuals (see Figure 1). Gene drives may be used to push traits into pest populations that render them less damaging (e.g., to immunize them to a human, livestock, or crop disease which they transmit) or to drive traits, such as sterility, that can cause the entire pest population to collapse.
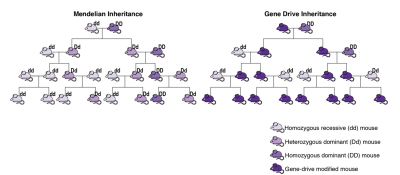
This figure shows the spread of a desirable gene mutation,
‘D,’ through a population of diploid organisms (having two
chromosomes);‘d’ is the original, undesirable copy of the
gene. With normal Mendelian inheritance, DD-type
organisms mating with dd-type organisms produce dD-type
organisms. But with gene drives, a DD-type with a gene
drive will always generate DD-type offspring (also with
gene drives), regardless of the mate’s type. This feature
means that the D mutation the population can be entirely
converted to DD-types by releasing only a small number of
individuals initially. Adapted from NASEM (2016a, Fig. 1-2).
Researchers are pursuing applications of gene drives to control a number of economically significant agricultural pests. These include Lepidopteran insects (among the most significant insect pests of corn, cotton, and soy, accounting for over two-thirds of U.S. crop value, U.S. Department of Agriculture, 2016), spotted wing Drosophila (one of the most significant pests of small fruit and berry producers in the United States, with potential crop losses of $718 million annually, Bolda, Goodhue, and Zalom, 2010), and Asian citrus psyllid (a devastating agricultural pest with damages likely exceeding $4 billion in Florida between 2006 and 2011, Hodges and Spreen, 2012). Other related work has explored using gene drives to control human disease vectors— such as mosquitoes transmitting malaria, dengue and Zika—and control invasive species that compromise biodiversity, such as invasive rodents and insects threatening endangered island birds. In spite of this potential and the demonstration of working gene drives in laboratory trials with caged organisms, no practical gene drive system has yet been created for an agricultural pest.
Even once a working gene drive system is created, there are significant questions about the risks, economics, and governance of such a technology. Because of its key feature—the evolutionary chain reaction—it may be impossible for deployers to precisely control who is affected by the release of a gene drive. Neighboring farmers, regions, and countries may experience unavoidable and irreversible impacts through the release of a gene drive by a single deployer. This raises questions for agricultural producers and the general public not only for risk assessment and domestic regulation but also international treaties, global trade, and how businesses profit from the technology.
The key attraction of gene drives is the possibility of permanently eliminating pest populations over wide areas. However, this source of attraction also poses challenges for commercialization: Area-wide pest control would benefit all parties in the area damaged by the pest, whether or not they paid for deployment costs. From an economist’s perspective, this means that gene drives have some attributes of a public good: Parties cannot be excluded from their benefits, and one party’s benefits do not come at the cost of another’s. Without institutions to incentivize cooperation, the private sector tends to underinvest in public goods because individual firms find it difficult to capture profits from the non-excludable benefits that public goods generate. With gene drives, the logic of public goods suggests the bulk of R&D in the technology will remain in the public sector. For the most part, this has been the case with prior approaches to area-wide pest control techniques, including the “sterile insect technique” (SIT) and classical biocontrol. SIT consists of sterilizing pests (via genetic modification or irradiation) and releasing them in large numbers to drive down the overall pest population. It differs from gene drive approaches in that population suppression via SIT may be reversed if continual releases of sterilized insects are not maintained. Gene drives, in contrast, may irreversibly alter or eliminate pest populations. This irreversible, invasive potential of gene drives is shared by classical biocontrol, which involves the intentional, permanent establishment of predators or parasites of a target pest (e.g., by finding and transplanting a predator from the pest’s native habitat). The impacts of biocontrol tend to be bimodal: They either yield dramatic impacts (for better or worse) or practically none at all, precisely because of their invasive potential.
As with SIT and biocontrol, gene drive releases would likely involve concentrated capital and operational costs and offer the possibility of diffuse, area-wide (or region-wide) benefits. This economic structure of gene drive development makes it more likely that deployments will come from the public rather than the private sector, as has been the case with SIT and biocontrol. The most enduring SIT success story—the elimination of the New World screwworm, a major pest of livestock, from North and Central America—involved substantial investment by the USDA in coordination with the governments of Mexico and Central American countries. Project expenses included constructing facilities for rearing and sterilizing screwworm and operational costs for running the facilities and for aerial deployments of the sterilized screwworm over wide regions. The benefits, estimated to be on the order of $800 million annually in reduced damages to U.S. agriculture, have easily exceeded these costs (Vargas-Terán, Hofmann, and Tweddle, 2005; Concha et al., 2006). It is difficult to imagine, given the large-scale centralized operation of this program, how private R&D investment would have been able to substitute for government investment in this case.
Cooperative efforts by farmers’ groups can also generate substantial investment in areawide pest control. The Pink Bollworm Eradication program provides an important example. This successful program involved a $121 million investment in private control efforts and areawide pest monitoring and pheromone applications from cotton growers in the southwestern U.S. and northern Mexico over five years. USDA complemented this private investment with an additional $35.3 million SIT program over the same period (APHIS, 2009).
To date, research on gene drives appears to exhibit similar public goods attributes. Most major advances in gene drive research have been made by researchers at universities, not by private firms. Researchers at Berkeley and the Massachusetts Institute of Technology possess the key patents for the CRISPR/Cas9 gene editing method (with an ongoing dispute over their competing claims; Cohen, 2017). And Oxitec, the biggest and most well-known firm using genetic engineering to control insect pests and disease vectors, has publicly stated its intention to not yet commercialize gene drives (Oxitec, 2016).
Oxitec’s decision not commercialize gene drives also likely relates to uncertainty about the risks and regulation of the technology, discussed below. As with their potential benefits, the risks of gene drives are also likely non-excludable. This creates the possibility of another, related economic phenomenon arising from the technology: negative externalities. These arise when the decision of one party (e.g., an individual grower or firm) collaterally damages another party (e.g., another grower or nearby resident), such that those damages are not accounted for in the decision of the first agent. In the case of gene drives, the deployers of the technology may not fully internalize possibly irreversible risks to public health or the environment in releasing the GM insects. These external risks may affect parties at multiple scales (e.g., other growers in an area where a gene drive has been released or countries sharing a border with a country whose government has approved a gene drive for wide-scale release).
Both the phenomena of public goods and negative externalities require some form of corrective action to achieve economic efficiency. For public goods, the most common instrument for correcting under-provision is taxation to fund public investment (e.g., the use of tax dollars to fund the USDA’s SIT screwworm suppression program). For negative externalities, corrective instruments vary depending on the nature of the external damages and the affected parties. As a hypothetical example, organic growers in a local area might lose their organic certification if invaded with GM gene drive insect pests that a large, neighboring non-organic grower plans to release. One corrective action would be for the organic growers to pay the non-organic grower up to the amount of damages they expect from the GM pest. This payment would constitute an opportunity cost to the non-organic grower of releasing the gene drive insects. Alternatively, if the non-organic grower were legally liable for damages to organic growers from the release, then this would be factored into the decision whether to release the gene drive. Either arrangement could yield economic efficiency, a concept in economics known as the Coase Theorem (after University of Chicago economist and Nobel Prize winner Ronald Coase). In reality, the ability of private agents to coordinate economically efficient outcomes is often much more complicated. At a local level, agricultural areas with strong growers’ associations may be more likely to resolve local public goods and externality issues related to area-wide pest control, including deployment of gene drives.
As with GM crops, the economics of gene drives relate closely to how they will be regulated and to the nature of the risks they pose. In the United States, some form of environmental or risk analysis of gene drives will be required for regulatory approval. The National Environmental Policy Act (NEPA) requires federal government actions to be reviewed for their environmental impacts. NEPA requires two levels of analysis: an environmental assessment (EA) and an environmental impact statement (EIS). An EIS is more extensive, time-consuming, and costly than an EA. The former is triggered by an EA, unless there is a “finding of no significant impact.”
Whether an EA or an EIS (or some other form of environmental evaluation) will be required for gene drives will depend on the U.S. regulatory authority responsible for the specific application. In the U.S. federal government, biotechnology governance is managed under the Coordinated Framework for the Regulation of Biotechnology, which distributes regulatory responsibility across existing, applicable laws and their implementing agencies: the USDA, the EPA, and the FDA. However, the unique properties of gene drives (especially including the CRISPR/Cas9 gene editing method) obfuscate which existing laws may apply to these technologies. Regulatory processes under the existing Coordinated Framework would therefore be determined on a case-by-case basis. In response to this regulatory uncertainty surrounding gene drives and other novel biotechnologies, the Coordinated Framework has been under review for possible revision since 2015. However, no public decisions have yet been made, and regulatory uncertainty persists.
Such regulatory uncertainty can increase private sector R&D costs. A 2016 report by the National Academies of Sciences, Engineering, and Medicine (NASEM) on GM crops reported estimates from industry that commercializing a new GM crop variety can directly cost at least $15 million and delay commercialization by 5–10 years, due to time involved gathering data for submitting an application and awaiting a regulatory decision. The substantial fixed costs from regulation likely inhibit smaller, less capitalized firms from innovating in this space (NASEM, 2016b, Ch. 6).
These regulatory costs and delays may be justified insofar as the level scrutiny is commensurate with the overall risks to society. Another 2016 NASEM report, on gene drives, addresses the question of risk in relation to potential benefits. Among the possible risks of gene drives raised by the report are the creation of ecological niches that could be filled by problematic competitors of the suppressed pest as well as the possibility that the target pest evolves some form of resistance to all or part of the gene drive (NASEM, 2016a). While the report did not attempt to monetize the expected benefits or risks of the technology due to its nascent stage of development, the NASEM committee recommended the use of ecological risk assessment (ERA) for evaluating unintended consequences of gene drive releases, in combination with a phased testing of novel gene drives. Ecological risk assessment is an approach to identifying and probabilistically quantifying the future potential impacts of gene drive releases. Phased testing consists of moving from laboratory-based testing to “staged field release,” followed by post-release surveillance. All of these phases are likely to generate scientific data for risk assessments.
ERA would go beyond the standard EA and EIS requirements of NEPA, due to ERA’s explicit focus on probabilistic quantification of possible outcomes—good and bad—under different policy alternatives (e.g., regulatory approval or denial of a gene drive application). In the case of gene drives, one motivation using ERA is the possibility of irreversible risks, such as the permanent, global replacement of a pest with an even more problematic gene drive strain of the organism. ERA would be suitable for identifying such irreversible risks and quantifying their likelihood.
Quantifying irreversible risks associated with gene drives would also facilitate useful economic analysis of their benefits and costs. Irreversible risk cannot be analyzed with usual methods of benefit-cost analysis (BCA) because conventional BCA does not recognize the potential value of waiting to learn additional information about such risks. An alternative economic criterion for assessing irreversible risks is option value, which can coherently compare the future benefits and costs of releasing a new technology, accounting for its irreversible impacts. The basic idea of option value is to account for the value of waiting to learn more about the benefits and risks of the technology before making the irreversible decision to release it into the environment. A plain-language overview of option value compared to BCA can be found in a report on BCA guidelines published by the Organisation for Economic Cooperation and Development (Pearce, Arkinson, and Mourato, 2006, Ch. 10).
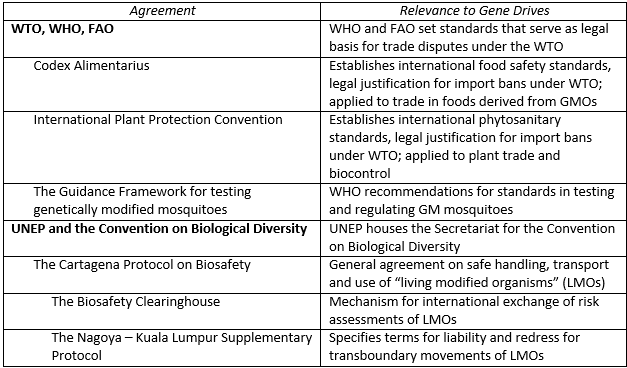
Acronyms: FAO = Food and Agriculture Organization, UNEP = United Nations Environment
Programme, WHO = World Health Organisation, WTO = World Trade Organisation
The economic externalities and public goods issues implicated in gene drive deployments also extend to international relations. Table 1 shows a number of international treaties, standards, and agreements that may be used in the international governance of gene drives. It remains to be seen how these agreements will be applied to gene drives (or whether new international agreements might be required to specifically address the technology). Transboundary movement of GM organisms (GMOs) or so-called “Living Modified Organisms” (LMOs, a term used frequently in international law) has historically been managed through food safety and phytosanitary standards adopted by the World Trade Organization. The principal standard applied to GMOs is the Codex Alimentarius, developed by the Food and Agriculture Organization (FAO) and World Health Organization (WHO). These standards are used by the WTO to evaluate the legality of import bans on GM food. In the case of gene drives used for agricultural pest control, the Codex Alimentarius could be used to evaluate cases where transgenic residues from an engineered insect were found on food.
However, in contrast to foods derived from GM crops, gene drives have special potential for direct transboundary movement and establishment. As noted above, the fact that gene drives are intended to spread makes them similar in some respects to classical biological control. International standards on biological control have historically been managed through the International Plant Protection Convention (IPPC) managed by the FAO. The IPPC in fact provides the phytosanitary standards used by the WTO to settle trade issues related to plant pathogen or pest introductions.
In addition to the WTO’s standards, the Convention on Biological Diversity (CBD) also has implications for how gene drives are handled under international law. While the United States has not ratified the CBD, the fact that all major U.S. trading partners have ratified the CBD makes it relevant to consider for U.S. agriculture. The Cartagena Protocol within the CBD is most relevant for gene drive deployment. The Cartagena Protocol issues standards and maintains a repository for LMO risk assessments through the Biosafety Clearinghouse. The protocol also addresses how damages for transboundary spread of LMOs are to be compensated. Rules for assessing such damages are being negotiated by CBD member countries via the Nagoya–Kuala Lumpur Supplementary Protocol on Liability and Redress, which CBD member countries are in the process of ratifying. For gene drives, this would mean that deployers of gene drives in countries who are parties to this protocol (hence excluding deployers in the United States) would be effectively liable for damages to other parties under this protocol as international law. Ratifying governments would be able to seek compensation and mitigation of damages from a gene drive deployer as long as that deployer was located in a ratifying country.
From the perspective of economic efficiency, these agreements are important as a means of partially correcting international externalities associated with gene drive releases. Without liability, a deployer would not internalize the risks to other parties associated with their decision to release (unless those put at risk paid the deployer not to deploy, in the vein of the Coase Theorem, discussed above). However, it deserves mentioning that the CBD only addresses risk liability associated with LMOs, not the public goods aspects discussed above. Efficient international instruments for correcting these externalities would also allow the deployer to capture some of the benefits to transboundary spread of the gene drive organism. Considering the example of successful international cooperation on the SIT screwworm program, such a mechanism could involve international public R&D investments in some gene drive systems (including integrated risk assessment components, as described above).
Many of these issues were discussed at a recent 2016 workshop hosted by the Genetic Engineering and Society (GES) Center and sponsored by the Organisation for Economic Cooperation and Development (OECD) with the North Carolina Biotechnology Center (GES, 2016). The focus of the workshop was on the precursors necessary for the international harmonization of regulations concerning GPM technologies, including gene drives. The two-day event involved participants from industry, NGOs, government, and academia. After presenting descriptions of biotechnology regulation across a number of countries (including the United States), participants engaged in a stakeholder mapping exercise with a number of hypothetical case studies of potential future deployments of gene drives. The exercises required participants to identify local and global stakeholders in different gene drive release scenarios. Once stakeholders had been identified for the scenario, they were categorized by the amount of power they had over the release decisions (e.g., authority under the law as well as financial resources) and how much interest or stake they had in the possible outcomes of the gene drive release. One of the conclusions of the workshop was that formal, effective stakeholder engagement processes will be necessary in the international governance of gene drives. This suggests that the when building gene drive governance into existing institutions, such as the WTO or the CBD, the extent and effectiveness of stakeholder engagement processes within those institutions should be considered.
Gene drives are radically different approach to the control of animal pests, invasive species, and disease vectors. A number of scientific, economic, and social questions are pertinent when considering whether to and how to adopt such an approach. Scientific questions include the technical feasibility of gene drives in terms of achieving their design objective, how the permanent removal or alteration of entire species will affect the broader ecosystems to which they belong, and the feasibility of reversing alterations to ecosystems in the event of adverse ecological consequences. Economic questions are how to weigh these uncertain benefits and risks, accounting for the possibility that gene drive deployment may be irreversible. The central social questions concern the relative value we place on pest and disease reduction benefits (economic or otherwise) and the possible adverse environmental consequences of gene drives. In addition, because gene drives are intended to spread, their impacts cannot be relegated to any one area, group of stakeholders, or country. This non-excludability of gene drive impacts provides a rationale, at a local and regional scale, for public support of applied gene drive research and ecological risk assessment, and for a strong role to be played by agricultural cooperatives and growers’ associations in supporting and monitoring gene drive deployments. At the international level, the non-excludability of impacts raises the possibility of international disputes over unilateral gene drive deployments, creating a need for effective international institutions for settling these disputes and internalizing the global consequences of countries’ domestic biosafety regulations.
Animal and Plant Health Inspection Service (APHIS). 2009. “Pink Bollworm Eradication Current Status.” Accessed on May 2, 2017. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/cotton_pests/downloads/PBW_prog_update2009.pdf
Bolda, M. P., R. E. Goodhue, and F. G. Zalom. 2010. “Spotted Wing Drosophila: Potential Economic Impact of a Newly Established Pest.” Agricultural and Resource Economics Update 13(3):5–8. Available online: http://giannini.ucop.edu/media/are-update/files/articles/v13n3_2.pdf
Cohen, J. 2017. “Round one of CRISPR patent legal battle goes to the Broad Institute.” Science Magazine News. Published 2/15/2017. American Association for the Advancement of Science (AAAS). Available online: http://www.sciencemag.org/news/2017/02/round-one-crispr-patent-legal-battle-goes-broad-institute
Concha, C., A. Palavesam, F. D. Guerrero, A. Sagel, F. Li, J. A. Osborne, Y. Hernandez, T. Pardo, G. Quintero, M. Vasquez, G. P. Keller, P. L. Phillips, J. B. Welch, W. O. McMillan, S. R. Skoda, and M. J. Scott. 2016. “A Transgenic Male-Only Strain of the New World Screwworm for an Improved Control Program Using the Sterile Insect Technique.” BMC Biology 14(1):72.
Genetic Engineering and Society Center (GES). 2016. Environmental Release of Engineered Pests: Building an International Governance Framework. Available online: https://research.ncsu.edu/ges/research-ges/oecd-crp-meeting
Hodges, A. W., and T. H. Spreen. 2012. Economic Impacts of Citrus Greening (HLB) in Florida, 2006/07–2010/11. EDIS #FE903, Food and Resource Economics Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainesville. Available online: http://www.crec.ifas.ufl.edu/extension/greening/PDF/FE90300.pdf
National Academies of Sciences, Engineering, and Medicine (NASEM). 2016a. Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values. Washington, D.C.: National Academies Press.
National Academies of Sciences, Engineering and Medicine (NASEM). 2016b. Genetically Engineered Crops: Experiences and Prospects. Washington, D.C.: National Academies Press.
Oxitec. 2016. “Academies of Sciences, Engineering, and Medicine recommendations on gene-drive and highlights why Oxitec’s self-limiting approach is the opposite of gene-drive method.” Published on 6/8/2016. Available online: http://www.oxitec.com/press-release-oxitec-supports-national-academies-sciences-engineering-medicine-recommendations-gene-drive/
Pearce, D., G. Atkinson, and S. Mourato. 2006. Cost-Benefit Analysis and the Environment: Recent Developments. Paris, France: OECD Publishing.
U.S. Department of Agriculture. 2016. Crop Values: 2015 Summary. Washington, D.C.: National Agricultural Statistics Service, February. Available online: https://www.usda.gov/nass/PUBS/TODAYRPT/cpvl0216.pdf
Vargas-Terán, M., H. C. Hofmann, and N. E. Tweddle. 2005. “Impact of Screwworm Eradication Programmes Using the Sterile Insect Technique.” In V. A. Dyck, J. Hendrichs, and A. S. Robinson, eds. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Berlin/Heidelberg: Springer-Verlag, 629–650.
Further details may be found in a volume on the Economics of Integrated Pest Management of Insects, forthcoming from CABI in 2018.